Shane Keniley, Ph.D. Candidate
Dr. Davide Curreli, Director of Research
December 16, 2021 1:00pm – 3:00pm
Multiscale Fluid Modeling of Direct-Current Plasma-Water Interactions
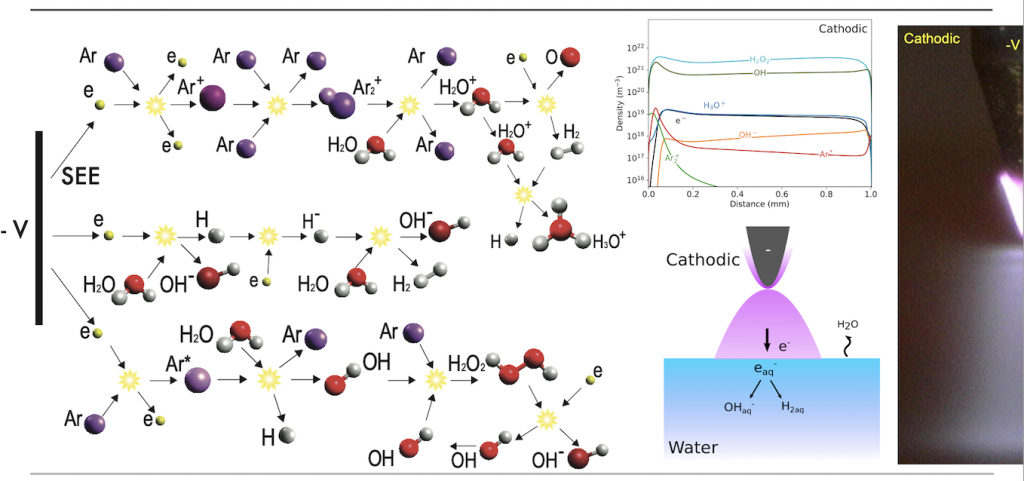
ABSTRACT: One of the most promising emerging fields in plasmas is that of plasma-water interactions. Electrons in the plasma facilitate the production of both short- and long-lived reactive oxygen and nitrogen species, which have been shown to disinfect surfaces, induce cancerous cell death, and open up reaction pathways for high value chemical synthesis. A plasma in direct electrical contact with water will induce all of these effects in both the gas and liquid phases. Charged and neutral species may transport through the liquid interface, which changes the chemical composition of both phases and forms a tightly coupled electro-chemical. These conditions are difficult to characterize both numerically and experimentally because of the disparate spatial and temporal scales at play: solvated electrons are expected to drive chemical reactions in a region only nanometers thick at the interface, and reactive species lifetimes range from microseconds to hours.
This work is focused on the multiscale modeling of a direct-current (DC) plasma with a liquid water counterelectrode. A new open source plasma chemistry software, Crane, was developed in the MOOSE finite element framework in order to study the detailed chemical reactions that exist in plasma-water systems. Crane was coupled to a MOOSE application dedicated to plasma transport, Zapdos, which was upgraded to facilitate the modeling of multispecies plasmas. Both codes were verified against known global and 1D plasma transport problems and compared to existing software. A fully coupled plasma-water interface model was developed using the combined software, including electron transport across the interface, neutral species solvation and evaporation, and tightly coupled chemical reaction networks in both plasma and liquid phases.
The coupled plasma transport and chemistry models allow for the analysis of the electronic and chemical structure of the plasma-water interface. Results show that the chemical composition of the water is dramatically affected by the polarity of the driven electrode in the plasma phase. During anodic plasma treatment, a strong electric field forms in the cathode fall region over the water surface, which facilitates the production of near-surface reactive oxygen species (ROS). These species, along with positive ions, dissolve into the water and lead to the production of hydrogen peroxide (H2O2(aq)), ozone (O3(aq)), and hydroxyl radicals (OH(aq)). In contrast, the primary species injected into the water during cathodic plasma treatment are the highly reactive solvated electrons (e(aq)), which react with and inhibit the accumulation of ROS.
Simulations were supported by optical emission spectroscopy and chemical probe measurements in an equivalent electrochemical cell. The numerical model predicted that the disparate H2O2(aq) concentrations seen between anodic and cathodic plasma operation are the result of solvated electrons degrading any H2O2(aq) before it is allowed to accumulate. Experiments carried out in support of this prediction demonstrated that the H2O2(aq) concentration increased as solvated electron scavengers were added, showing strong agreement with simulation results. These results emphasize the tightly coupled nature of the plasma- water interface and may be used to inform future experimental work on water disinfection and chemical production using plasma electrolysis systems.